VintageTexas Sunday Cyclopedia of Wine: Acidity
Think of acidity and fruit, and your mind immediately goes to the tart and titillation of lemon juice. But, what would wine be without acidity? It would be a flat, non-refreshing beverage. Acidity is often underrated in its role in many of wine’s qualities from its mouthfeel to its ageability.
Acidity is a natural and essential component of wine that, on a very basic level, underwrites a wine’s zestiness and liveliness that has secured its place as the beverage of choice and placement on the table with many of the world’s finest foods. Equally important is the role of acidity in the aging potential and longevity of wines, especially white wines that lack the tannins commonly found in red wines. Furthermore, in sweet and semi-sweet wines, acidity reduces the apparent sweetness of these wines on the palate and the cloying tendency in these styles of wine. Across the board, whether it’s German Riesling, French Chablis or Bordeaux, or New Zealand Sauvignon Blanc, the natural acidity of wines (dominated by tartaric acid) plays a key role in just about every aspect of wine that we love so much.
On the other side of the balance sheet, the acidity arising from the formation of acetic acid resulting from excessive oxidation of the wine and bacterial spoilage. This is commonly observed first observed as a burning sensation in the back of the mouth before it brings the obvious and commonly known smell of vinegar to the nose. More after the jump!
Terms that define a wine’s acidity include:
- Total acidity which refers to all of the acids derived from the grapes and the winemaking process (tartaric, malic, lactic, acetic, etc.)
- Volatile acids are those such as acetic acid that will volatize/evaporate during heating or boiling.
- Fixed acids are those that remain in the wine after boiling are tartaric, malic, citric, lactic and succinic acid.
- pH is a measure of acidity from any and all acids present in a wine. A pH of 7 is considered neutral and anything less than 7 is acidic. Most wines are most stable and produced in the pH range of 3 to 4.
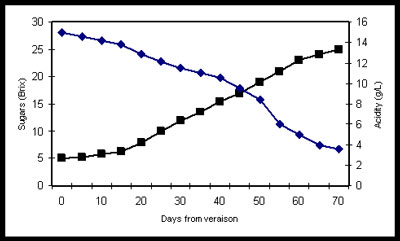
In warmer climates or in warmer years, grapes tend to ripen further which tends to produce grapes with higher sugar content and lower natural acidity. This can result in wines having higher alcohol content and lower in acidity (higher pH). If this situation presents itself, the winemakers will often make adjustments (additions of acid during the winemaking process) in an attempt to reduce pH to improve the zestiness of the wine on the palate and improve it stability for aging and prevent spoilage in the bottle. If the grapes are overripe, too much acid addition can yield a harsh, unnaturally acidic feeling on the palate. Conversely, in cooler climates or cooler years, grapes have higher natural acidity dominated by tartaric and malic acid. The winemaker can use a secondary fermentation technique called malolatic fermentation to convert the harsher malic acid to the more pleasant lactic acid.
Further references on the acidity of wine are given below:
http://www.wineperspective.com/the_acidity_of_wine.htm
http://en.wikipedia.org/wiki/Acids_in_wine
http://winemaking.jackkeller.net/acid.asp
http://www.thewinedoctor.com/advisory/tastecomponents.shtml




Be the first to comment